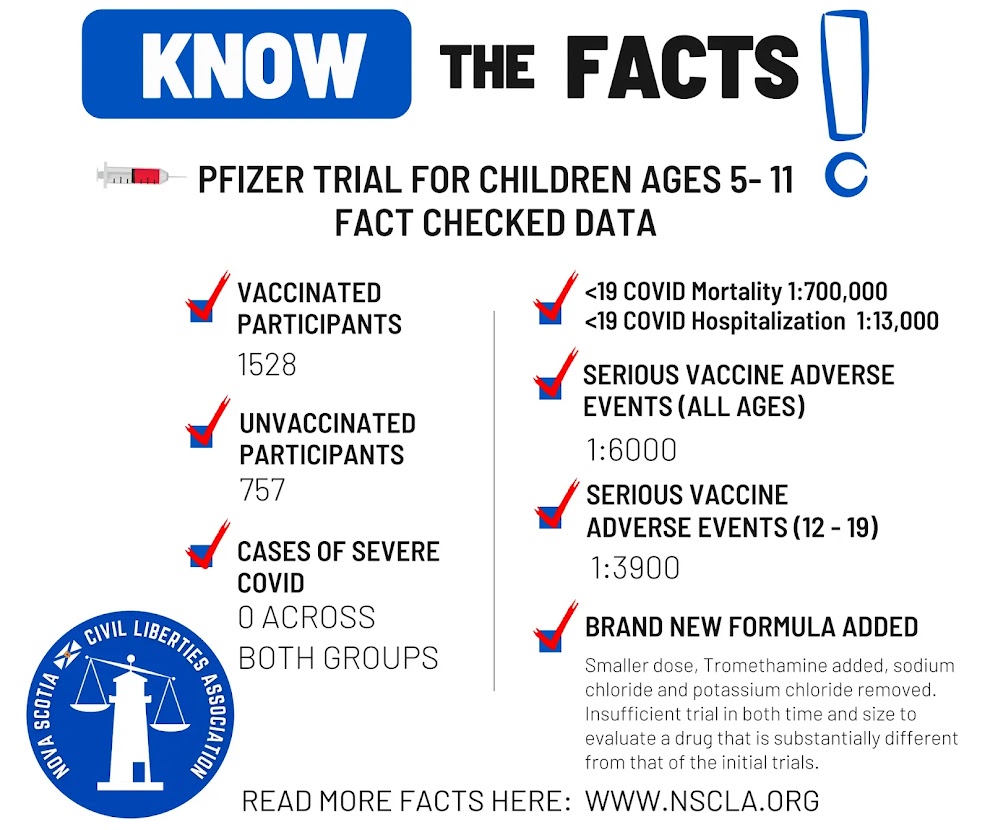
Pfizer recently completed their trials for children aged 5-11 and submitted to Health Canada for authorization. This application was approved on November 19, and we can expect vaccination to begin in short order. However, even a surface overview of the documentation produces very concerning results.
There were just 2285 children included in the study, of which just 1528 received the vaccine (757 received a placebo). Any adverse event that occurs less frequently than 1:1500, such as myocarditis which occurs at a rate of about 1:5000 in the 12-17 male demographic, would not be picked up by a trial that size. The entire trial only captured a total of 19 symptomatic Covid cases (16 in the placebo group, 3 in the vaccinated group). Not a single case was serious, all were mild. All the study confirms is that Covid generally only causes mild disease in children 5-11, and the vaccine reduces the occurrence of mild disease.
We already know from existing data that children 5-11 are at very low risk from Covid. As of November 8, 2021 Canadian data confirms those under 19 are hospitalized at an annualized rate of around 1:13000 people (8,000,000 people, 1899 hospitalizations over 19 months, only 57% of which were actually directly due to COVID) and risk of death is just 0.0048% (17 deaths from 359000 lab confirmed cases), or an annualized death rate of about 1:700,000.
The size and scope of the trial data is insufficient to determine the risk the vaccine poses, and there is no discernible benefit as this age group is at no statistical risk from Covid itself. Beyond this, the vaccine that has been approved for ages 5-11 is a different formulation to what was approved in the original clinical trials. It's one third of the dose an adult receives and as per Pfizer:
"To provide a vaccine with an improved stability profile, the Pfizer-BioNTech COVID-19 Vaccine
for use in children 5-11 years of age uses tromethamine (Tris) buffer instead of the phosphatebuffered saline (PBS) as used in the previous formulation and excludes sodium chloride and
potassium chloride."
A shockingly small trial combined with a substantially changed formula and dosage regimen coupled with the knowledge of the negligible risk Covid poses to this age group and the evidence that adverse events, especially myocarditis, seem to occur at an increasing rate the younger the age should give pause to anyone, regardless of where you fall on the ideological spectrum. It is the position of the NSCLA that the risk-benefit analysis overwhelmingly rejects widespread vaccination within this age group. We believe it should only be made available to those deemed high risk due to co-morbidities, and under no circumstances should any vaccine mandates apply to this age group. The vast majority of this age group is quite likely to be at far greater risk of a serious adverse event such as myocarditis from the vaccine, than any negative outcome from Covid. Using children as human shields to protect adults by vaccinating them to theoretically reduce the potential of spread, is immoral and unethical on every conceivable level. These actions offer no benefits to the child and put them at risk of vaccine injury. This must not happen.