For a disease that has been characterized as being the most deadly and impactful of our collective lifetimes, potential treatments for COVID-19 have been disturbingly contentious. Common sense suggests that any potential treatments which could help reduce strain on healthcare systems and ultimately reduce suffering and death would be not only welcomed, but rigorously investigated and used if there is even a hint of potential for positive outcomes. Astonishingly, the opposite is what has widely occurred, at least in western society. Any potential treatments from existing medications have been fiercely denounced by Government's and media, typically citing dangers of the unknown, lack of information, and often downright smear campaigns (seriously just Google "Ivermectin" and read the top results). However, there appears to be little appetite to investigate any further, which creates immense skepticism and distrust as the logic runs counter to common sense in the middle of a pandemic. Why is there so much rigor behind rolling out vaccines so quickly, but the opposite appears to be true with regards to investigating the validity of treatments using cheap, widely available drugs?
Next, we lay out the science around existing potential treatments for COVID-19 to see if there is any validity to their efficacy. We also discuss the new pills from Pharma recently approved for COVID-19 treatment.

Ivermectin
Given the attention this drug has received, it's hard to start anywhere else. First, let's be clear what we are discussing. Is Ivermectin horse medicine? Ivermectin Liquid for horses is formulated for administration by stomach tube (nasogastric intubation) or as an oral drench. The recommended dose is 200 µg of ivermectin per kilogram of body weight. Each mL contains sufficient ivermectin to treat 50 kg of body weight; 10 mL will treat a 500 kg horse. For humans, Mayo Clinic suggests a similar dosage as a horse receives per kg of body weight (same as 1mg per 5kg body weight). It's important to note that horses weigh significantly more than humans so taking a dose meant for a horse could indeed be harmful. Ivermectin is an FDA approved drug for use in humans to treat a variety of parasitic infections including parasitic worms, hookworm and whipworm. It may also be used as an effective treatment for a wide range of other conditions and as a treatment of onchocerciasis, intestinal strongyloidiasis and onchocerciasis or river blindness. The antiviral activity of Ivermectin has been shown against a wide range of RNA and DNA viruses, for example, dengue, Zika, yellow fever, and others.
As far as its efficacy for treatment of COVID-19, there is no shortage of data. A small study (randomized, double-blind, placebo-controlled trial) from early 2021 concluded that virologic clearance occurred about 25% sooner compared to the placebo group.
Another study published in September 2021 did a meta analysis of over 20 trials and confirmed notable reductions in COVID-19 fatalities, with a mean 31% relative risk of mortality vs. controls. During mass Ivermectin treatments in Peru, excess deaths fell by a mean of 74% over 30 days in its ten states with the most extensive treatments.
Finally, we have this extensive review of the impact Ivermectin had on the reduction of cases in parts of India.
The real question is, given the mountain of evidence that suggests Ivermectin is very effective against COVID-19, combined with it being incredibly safe, readily available and cheap (circa $2-$3 per dose), what possible reason could there be to smear this Nobel winning drug and restrictions on using it anywhere in the western world?
Monoclonal Antibodies
This treatment, brought into prominence due to its widespread use in Florida, is also approved for use in Canada. Unfortunately, it's not widely used so far. Simply put, monoclonal antibodies are synthetic antibodies made to target a specific disease to help fight an infection of that disease. The treatment is given intravenously. Monoclonal antibodies were approved for use in Canada in late 2020, with an initial delivery of 26000 doses, the bulk of which were shipped directly to provinces. Unfortunately they don't appear to have been put to effective use.
Freelance investigative reporter Linda Pannozzo outlines in fantastic detail, the monoclonal antibody situation in Nova Scotia. There are lots of reasons given as to why they haven't been widely used, including the logistics of setting up clinics where COVID positive patients can receive the treatment. However, I doubt families are terribly sympathetic to logistical challenges when life saving treatments are available but simply not being used.
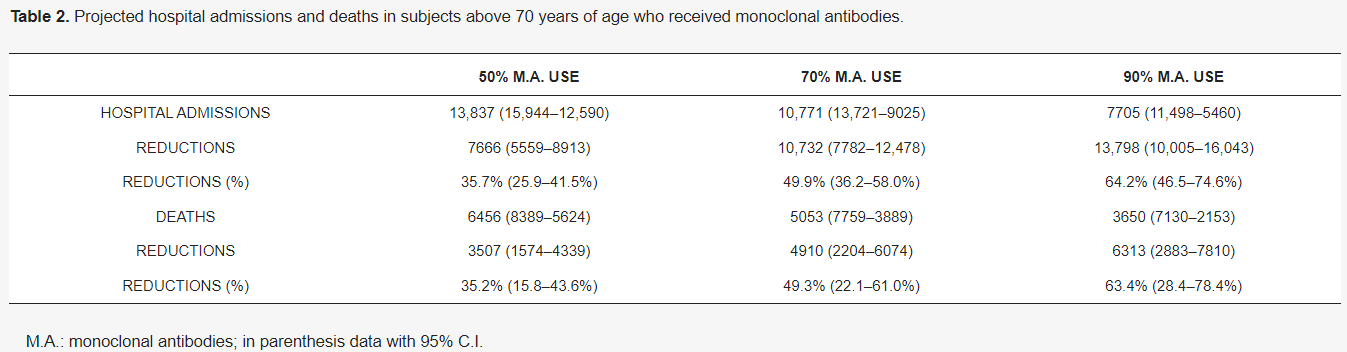
A study published in October 2021 using data from Italy estimates that both hospitalizations and deaths in COVID positive patients aged 70+ could be reduced by 35%-64% if monoclonal antibodies were used in 50%-90% of patients. How many Nova Scotian lives could have been saved if this treatment was used effectively over the last year? Someone needs to answer for that.
Molnupiravir & Paxlovid
These are Merck and Pfizer's newly introduced COVID treatment pills which are likely soon to be approved for use in Canada and the US; already approved in the UK.
Molnupiravir from Merck is a 5 day course of treatment where the patient takes 8 pills daily. Unvaccinated patients with mild or moderate cases of Covid who took Molnupiravir within five days of developing symptoms had about a 50% reduction in hospitalization and death rates, compared to those who received a placebo pill. The cost to treat each patient will be $700USD.
Paxlovid by Pfizer is a protease inhibitor. The course of treatment includes taking 4 Paxlovid pills daily for 5 days combined with 2 pills of a common HIV drug called ritonavir. According to Pfizer, this treatment reduces the risk of hospitalization or death in high-risk patients by 89%. The treatment costs $529.
Watch Dr John Campbell give an in depth pharmacological comparison of the mechanism by which Ivermectin and Paxlovid both inhibit COVID-19 viral replication
Vitamin D
A study published in October 2021 concludes a theoretical point of zero mortality could exist if everyone achieved a Vitamin D blood level of 50.7ng/ml, with mortality decreasing considerably at 30ng/ml. Thus, daily vitamin D3 supplementation in the range of 4000 to 10,000 units (100 to 250 µg) needed to generate an optimal vitamin D3 blood level in the range of 40–60 ng/mL has been shown to be completely safe when combined with approximately 200 µg/mL vitamin K2.